These rules apply to all marketing communications for food products and should be
read in conjunction with the relevant legislation:
1) Regulation (EU) No. 1169/2011 of the European Parliament and of the Council of 25 October, 2011, on the provision of food information to consumers as amended, including Commission Implementing Regulation (EU) No. 1337/2013 and its implementing legislation in Ireland, European Union (Provision of Food Information to Consumers) Regulations, 2014 (S.I. No. 556/2014).
2) Regulation (EC) No. 1924/2006 on nutrition and health claims made on foods (the EU Regulation) together with the amending legislation. They apply to all marketing communications for food products.
The EU Regulation on nutrition and health claims is mandatory and seeks to protect consumers from misleading or false claims. It came into force in July, 2007, but was subject to a series of complex transitional periods (see www.fsai.ie). Specific conditions of use associated with authorised health and nutrition claims are determined at a European level. The EU Register of nutrition and health claims (the EU Register) lists all authorised nutrition and health claims as well as non-authorised health claims that have been rejected.
Some transitional periods still apply, for instance, those affecting trademarks or brand names in use prior to 1 January, 2005. In addition, there are certain claims which the European Commission has placed “on hold” whilst discussions take place on the best way forward for these types of claims. Claims that are on hold are subject to an extended transition period and are still permitted for use.
The ASAI encourages advertising industry stakeholders to take advice on the effect
of the EU Regulation.
Definitions
8.1
For the purposes of the rules in this Section:
(a) “Nutrition claim” means any claim which states, suggests or implies that a food has particular beneficial nutritional properties due to:
(i) the energy (calorific value) it provides; provides at a reduced or increased rate; or does not provide; and/or
(ii) the nutrients or other substances it contains; contains in reduced or increased proportions; or does not contain.
(b) “Health claim” means any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health.
(c) “Reduction of disease risk claim” means any health claim that states, suggests or implies that the consumption of a food category, a food or one of its constituents significantly reduces a risk factor in the development of a human disease.
(d) HFSS Food is a sub-category of food that is deemed high in fat, sugar and/or salt by the application of the Nutrient Profile model detailed in Appendix I to this Section of the Code.
8.2
References to food apply also to non-alcoholic beverages and food supplements.
8.3
Marketing communications for food should not make reference to consumer taste or preference tests in any way that might imply statistical validity if there is no such validity, and should not use scientific terms to ascribe validity to an advertising claim which is not valid.
Diet and Lifestyle
8.4
Marketing communications for food should not encourage or condone excess consumption. They should not encourage an unhealthy lifestyle or unhealthy/unbalanced eating or drinking habits.
8.5
Marketing communications for food should not show people who choose a healthy active lifestyle in a negative manner.
8.6
Marketing communications for food should not disparage good dietary practice or the selection of options that accepted dietary opinion recommends should form part of the average diet.
8.7
Marketing communications for food representing any material characteristics of the product, including size and content, should be accurate and should not mislead consumers concerning any of those characteristics, or the intended use of the product.
Nutrition and Health Claims
8.8
Only nutrition claims listed in the updated Annex of the EU Regulation (as reproduced in the EU Register) may be used in marketing communications.
Only health claims listed as authorised in the EU Register, or claims that would have the same meaning to the consumer, may be used in marketing communications.
8.9
Marketing communications that contain nutrition or health claims should be supported by documentary evidence substantiating that they meet the conditions of use associated with the relevant claim, as specified in the EU Register.
8.10
Claims should be presented clearly and without exaggeration.
8.11
References to general benefits of a nutrient or food for overall good health or health-related well-being are acceptable only if accompanied by a specific authorised health claim.
8.12
A comparison may only be made between foods of the same category, taking into consideration a range of foods of that category. The difference in the quantity of a nutrient and/or the energy value shall be stated and the comparison shall relate to the same quantity of food.
8.13
Comparative nutrition claims shall compare the composition of the food in question with a range of foods of the same category, which do not have a composition which allows them to bear a claim, including foods of other brands.
8.14
The following are not acceptable in marketing communications for food products:
(a) Claims that state or imply health could be affected by not consuming a food.
(b) Claims that state or imply a food prevents, treats or cures human disease. Reduction of disease risk claims are acceptable if authorised by the European Commission.
(c) Health claims that refer to the recommendation of an individual health professional. Health claims that refer to the recommendation of an association are acceptable only if that association is a health-related charity or a national representative body of medicine, nutrition or dietetics.
(d) References to changes in bodily functions that could give rise to, or exploit, fear in the audience.
(e) Claims of a nutrition or health benefit that give rise to doubt about the safety or nutritional adequacy of another product.
(f) Health claims that refer to a rate or amount of weight loss.
8.15
Marketing communications for food should not mislead as to the nutritive value of any food.
Children
8.16
In addition to all other rules in this Section, marketing communications for food and beverages addressed to children:
(a) Should not denigrate a healthy lifestyle or encourage an unhealthy lifestyle or unhealthy eating or drinking habits; marketing communications representing mealtime should clearly and adequately depict the role of the product, where appropriate, within the framework of a balanced diet; snack foods should be clearly represented as such, and not as substitutes for meals.
(b) Should not mislead children as to the potential benefits from consumption of the product, either physically, socially or psychologically.
8.17
Marketing communications should not disparage good dietary practice or the selection of options that accepted dietary opinion recommends should form part of the average diet.
8.18
Marketing communications should not condone or encourage poor nutritional habits or an unhealthy lifestyle in children.
Placement of marketing communications for HFSS products in non-broadcast media
8.19
Marketing communications for HFSS food should not be directed or targeted at children under 15 through the selection of media or the context in which they appear.
8.20
No medium should be used to advertise HFSS products if more than 50% of its audience is under 15 years of age.
Media specific rules
8.21
Where a marketing communication for HFSS is permissible, it shall be subject to media specific placement rules, including maximum thresholds for each medium. The details for each media are set out in the Guidance Note on High Fat, Salt and Sugar (HFSS) Food and Non-Alcoholic Beverages here.
Locations
8.22
Locations primarily used by children shall be free from all forms of marketing communication for HFSS foods. Examples of such settings include registered crèches, pre-schools, nurseries, family and child clinics, paediatric services, schools, dedicated school transport, playgrounds and youth centres.
Promotional offers
8.23
Marketing communications featuring a promotional offer should be
prepared with a due sense of responsibility.
8.24
Marketing communications, in non-broadcast media for HFSS food products, that are targeted at children should not include promotional offers or a competition, subject to the exception listed below:
(i) Point of sale displays, packages, wrappers, labels, tickets, timetables and menus.
8.25
Additionally, for children under 16:
(a) Except those for fresh fruit or fresh vegetables, marketing communications should not seem to encourage children to eat or drink a product only to take advantage of a promotional offer: the product should be offered on its merits, with the offer as an added incentive.
(b) Marketing communications featuring a promotional offer should ensure a significant presence for the product.
(c) Marketing communications featuring a promotional offer linked to a food product of interest to children should avoid creating a sense of urgency or encouraging the purchase of an excessive quantity for irresponsible consumption.
(d) Marketing communications should not encourage children to eat more than they otherwise would.
(e) Marketing communications for collection-based promotions should not seem to urge children or their parents to buy excessive quantities of food.
Licensed Characters and Celebrities
8.26
(a) Licensed characters and celebrities popular with children should always be used with a due sense of responsibility.
(b) (b) Marketing communications, in non-broadcast media for HFSS
food products, that are targeted at children should not include licensed characters or celebrities popular with children, subject to the exceptions listed below:
(i) Point of sale displays, packages, wrappers, labels, tickets, timetables and menus;
(ii) The prohibition does not apply to advertiser-created equity brand characters (puppets, persons or characters), which may be used by advertisers to sell the products they were designed to sell.
(iii) Licensed characters and celebrities popular with children may present factual and relevant generic statements about nutrition, safety, education or similar.
HFSS Sponsorship arrangements
8.28
These rules apply to all forms of commercial sponsorship, involving HFSS food, of activities or events of any kind.
8.29
The restrictions will not extend to corporate identities, trading names, or masterbrands.
8.30
No sponsorship involving HFSS food will be permitted for any other setting dedicated to use by children of primary school age.
8.31
No sponsorship involving HFSS food will be permitted of events of particular appeal to children of primary school age.
8.32
Existing sponsorship contracts and agreements which otherwise would be in breach of the code will be permitted to continue until they expire.
(See Effective Date for implementation date of rules 8.28 – 8.32)
Nutrition and Health Claims
8.33
Claims referring to children’s development and health are acceptable if authorised by the European Union (see Section 8.8).
Pressure to Purchase
8.34
Although children might be expected to exercise some preference over the food they eat or drink, marketing communications should be prepared with a due sense of responsibility and should not directly advise or ask children to buy or to ask their parents or other adults to make enquiries or purchases for them.
Placement of marketing communications for HFSS products in non-broadcast media
8.35
Where a marketing communication for HFSS is permissible, it shall be subject to media specific placement rules, including maximum thresholds for each medium. The details for each media are set out in the Guidance Note on High Fat, Salt and Sugar (HFSS) Food and Non-Alcoholic Beverages here.
Food Supplements, including Vitamins and Minerals
8.36
Advertisers should ensure that claims for dietary supplements and other vitamins and minerals comply with the requirements of the EU Regulations.
8.37
Marketing communications that contain nutrition or health claims should be supported by documentary evidence to show they meet the conditions of use associated with the relevant claim as specified in the EU Register.
8.38
Marketing communications should not suggest or imply that a well-balanced diet needs to be augmented by vitamins or minerals on a regular basis. Advertisers may offer supplements as a safeguard and may refer to the vitamin and mineral content of a particular product but should not suggest that there is a widespread vitamin or mineral deficiency. Marketing
communications should not imply that supplements will guard against dietary deficiency, elevate mood or enhance performance and supplements should not be promoted as a substitute for a healthy diet. Marketing communications should not claim that a food supplement is capable of preventing, treating or curing disease.
8.39
Marketing communications may promote vitamin and mineral supplementation to certain categories of people, e.g. those who eat nutritionally inadequate meals, the elderly, children and adolescents, convalescents, athletes in training, those who pursue physically very active occupations or recreations, women of child-bearing age and dieters.
8.40
Although there may be some depletion of vitamin stores during illness, a marketing communication should not suggest that the replacement of such vitamins will influence the speed or extent of recovery. The prescribing of vitamins and minerals in such cases is a matter for a doctor and self-medication should not be encouraged.
Infant and Follow-on Formula
8.41
These rules should be read in conjunction with the relevant legislation including the ‘European Communities (Infant Formulae and Follow On Formula) Regulations, 2007’ (S.I. No. 852/2007).
8.42
Marketing communications for infant formula are prohibited unless they appear in scientific publications, or are for the purposes of trade before the retail stage, or are a publication for which the intended readers are not the general public.
8.43
Marketing communications should not confuse between infant formula and follow-on formula.
8.44
Marketing communications for follow on formula addressed to the general public should:
(a) Be designed to provide the necessary information about the appropriate use of the products so as not to discourage breast-feeding.
(b) Not use the terms ‘humanised’, ‘maternalised’, ‘adapted’ or similar terms.
Appendix I
Nutrient Profiling Model
For the purpose of this Code, the Nutrient Profiling Model adopted in the BAI General Communications Code (1st June 2017) based on the model developed by the UK Food Standards Agency should be used to assess whether commercial communications are for a product or service that is high in fat, salt or sugar and therefore subject to restrictions and regulation. There are three steps to working out the overall score of a food or drink.
1. Work out total ‘A’ points
A maximum of ten points can be awarded for each nutrient.
Total ‘A’ points = (points for energy) + (points for saturated fat) + (points for sugars) +
(points for sodium).
The following table indicates the points scored, depending on the amount of each nutrient in 100g of the food or drink:
Points allocation ‘A’ Nutrients
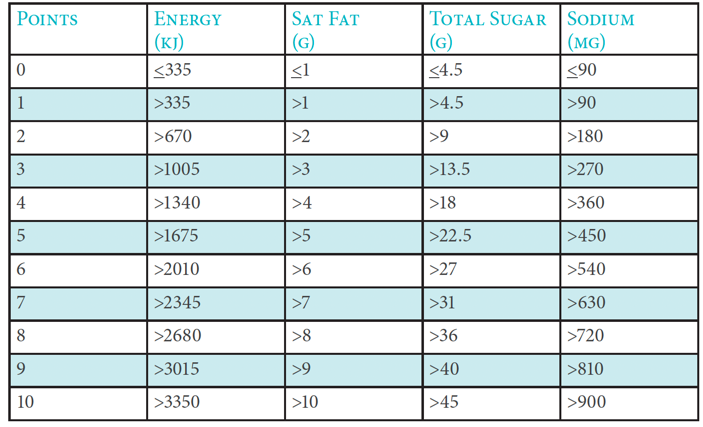
If a food or drink scores 11 or more ‘A’ points then it cannot score points for protein unless it also scores 5 points for fruit, vegetables and nuts.
2. Work out total ‘C’ points
A maximum of five points can be awarded for each nutrient/food component.
Total ‘C’ points = (points for % fruit, vegetable & nut content) + (points for fibre [either NSP or AOAC]) + (points for protein)
The following table indicates the points scored, depending on the amount of each nutrient/food component in 100g of the food or drink:
Points allocation ‘C’ Nutrients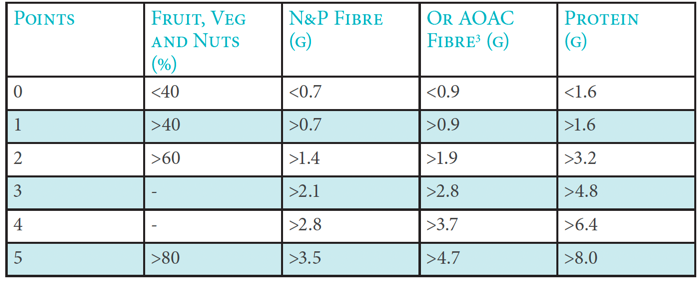
3. Work out overall score
If a food scores less than 11 ‘A’ points then the overall score is calculated as follows:
—Total ‘A’ points (energy + saturated fat + sugars + sodium) minus total ‘C’ points (fruit, veg and nuts + fibre + protein)
If a food scores 11 or more ‘A’ points but scores 5 points for fruit, vegetables and nuts then the overall score is calculated as follows:
—Total ‘A’ points (energy + saturated fat + sugars + sodium) minus total ‘C’ points (fruit, veg and nuts + fibre + protein)
If a food scores 11 or more ‘A’ points, and less than 5 points for fruit, vegetables and nuts, then the overall score is calculated as follows:
—Total ‘A’ points (energy + saturated fat + sugars + sodium) minus total points for fibre + points for fruit, vegetables and nuts (not allowed to score for protein)
A food is classified as ‘less healthy’ where it scores 4 points or more and is subject to the restrictions in the Code.
A drink is classified as ‘less healthy’ where it scores 1 point or more and is subject to the restrictions in the Code.
The full Technical Guidance and the Nutrition Profile Certification (which is the HFSS self-declaration certificate) are available here: www.asai.ie/npm.
3 One or other of the dietary fibre columns should be chosen to show the fibre content of the food or beverage was calculated by the manufacturer